Q1. When 50g of sugar is dissolved in 100mL of water, there is no increase in volume. What characteristic of matter is illustrated by this observation?

Q2. Why do trees acquire more leaves during summer?

Q3. Why do the doctors advise to put strips of wet cloth on the forehead of a person having high fever?

Q4. Which gas is called dry ice? Why?

Q5. Alka was making tea in a kettle. Suddenly she felt intense heat from the puff of steam gushing out of the spout of the kettle. She wondered whether the temperature of the steam was higher than that of the water boiling in the kettle. Comment.


Q6. Give two ways in which melting points and boiling points can be useful.

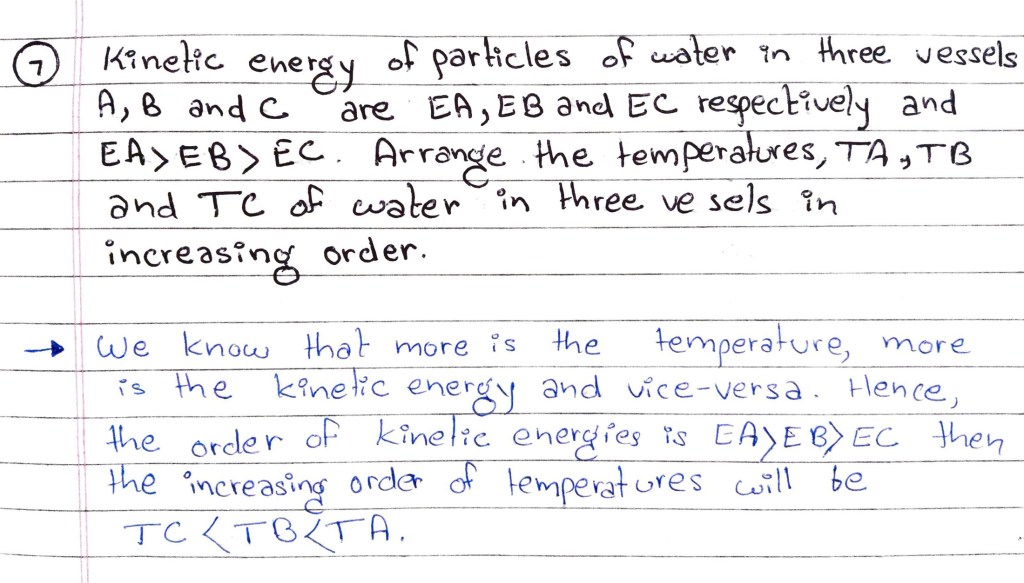
Q7. Kinetice energy of particles of water in three vessels A, B and C
are EA, EB and EC respectively and EA>EB>EC. Arrange the
temperatures, TA, TB and TC of water in the three vessels in increasing order.
Q8. Why do the gases exert more pressure on the walls of the container than the solids?

Q9. Substance ‘A’ has high compressibility and can be easily liquefied. It can take up the shape of any container. Predict The nature of the substance. Enlist four properties of this state of matter.

Q.10 Suggest an activity to show that the rate of diffusion of liquid decreases with increase in density of the liquid.

Q11. Discuss the various factors which affect the rate of evaporation. Latent heat of evaporation of two liquids A and B is 100J/kg and 150J/kg respectively. Which one can produce more cooling effect and why?

